How does the photoelectric effect work?

 The photoelectric effect is not new to scientists. Its roots can be found back in the 19th century.
In 1839, Alexander Becquerel observed that an electrode in a conductive solution emitted electrons when exposed to visible light.
The photoelectric effect is not new to scientists. Its roots can be found back in the 19th century.
In 1839, Alexander Becquerel observed that an electrode in a conductive solution emitted electrons when exposed to visible light.
Forty Eight years later, in 1887, Heinrich Hertz observed that when electromagnetic (EM) waves hit a metal surface, it emitted electrons. He published his results but never experimented enough on it.
During the next years, several scientists, such as JJ Thomson and
Von Lenard, involved in the research of this strange effect, but Albert Einstein was the one to describe what really happens. He also won his only Nobel prize for that!
Now, lets see what is it all about:
In a few words, the photoelectric effect refers to the emission of electrons from metal surfaces when hit by an electromagnetic wave (sunlight is one of those).
Ok, Now here is how it works:
Before Einstein, scientists could not decide what the nature of light was. Then, the experiments made on the photoelectric effect revealed something really impressive.
What was it?
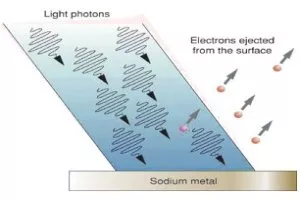 Well, if light was a wave, then the higher its intensity the more electrons should have been emitted. Whereas, if it was a "bunch" of particles, then the only factor counting would be its frequency. Remember that the colour of light depends on its frequency.
The truth is that it has the characteristics of both waves and particles at the same time. It just behaves in either way in different situations.
Well, if light was a wave, then the higher its intensity the more electrons should have been emitted. Whereas, if it was a "bunch" of particles, then the only factor counting would be its frequency. Remember that the colour of light depends on its frequency.
The truth is that it has the characteristics of both waves and particles at the same time. It just behaves in either way in different situations.
So, Albert Einstein told that light was transfered in "packets" of energy, later called photons, in order to explain this strange phenomenon. Each photon transfers almost all of its energy to one electron in order to "kick" it out of the metal surface.
That means that they need to give enough energy to do the job. Considering that the energy contained in each of them depend only on the frequency of incoming light, we see that:
- Not all frequencies (colour of light) are suitable
The higher the frequency, the more energy each electron will carry after its ejection.
- The intensity or direct/indirect radiation do not affect the ejection of electrons
BUT they affect the amount of the ejected electrons.
Nowadays, this effect has a great variety of applications, including the
photovoltaic cells.
P.S. The photoelectric effect observation was a key point of the
solar energy history. Thus, this description is adapted to the needs of the general concept of the
green energy production. Here is a more physics-point-of-view of the photoelectric effect.





 The photoelectric effect is not new to scientists. Its roots can be found back in the 19th century.
In 1839, Alexander Becquerel observed that an electrode in a conductive solution emitted electrons when exposed to visible light.
The photoelectric effect is not new to scientists. Its roots can be found back in the 19th century.
In 1839, Alexander Becquerel observed that an electrode in a conductive solution emitted electrons when exposed to visible light. Well, if light was a wave, then the higher its intensity the more electrons should have been emitted. Whereas, if it was a "bunch" of particles, then the only factor counting would be its frequency. Remember that the colour of light depends on its frequency.
The truth is that it has the characteristics of both waves and particles at the same time. It just behaves in either way in different situations.
Well, if light was a wave, then the higher its intensity the more electrons should have been emitted. Whereas, if it was a "bunch" of particles, then the only factor counting would be its frequency. Remember that the colour of light depends on its frequency.
The truth is that it has the characteristics of both waves and particles at the same time. It just behaves in either way in different situations.
